Evolution rests on the chance-based idea that raw matter plus undirected energy creates increasingly complex and ordered systems. This theory flies against the now-famous second law of thermodynamics, which states that every real system or process in nature (unless artificially constrained otherwise) tends to decrease in complexity and eventually decay. Also known as the law of entropy, this law of increasing disorganization has never been violated nor disproven. Dr. John Morris at ICR describes entropy as “a measure of the state of randomness or disorder in a system. While the total amount of energy always remains the same, the usefulness of that energy spontaneously degrades as the process proceeds, i.e., its entropy increases. Things are becoming less ordered and less energetic all the time. This is the opposite of evolution, which states that things become more complex through time, as molecules evolved into people.” [1] Any homeowner knows houses fall apart over time, and new bathrooms don’t remodel on their own. This law applies not only to physical matter but also extends into other fields such as information theory.
Though science classes mention the law of entropy, they blunt its impacts to evolution through appeals to ice crystals and seeds. They argue that water spontaneously forms into organized ice crystals, all six-sided and with no two alike. Likewise, seedlings pop up when the sun lingers in the sky. One fails to see how removing energy from water (which makes it cold) qualifies as a relevant example, since Darwin and others expect that lightning strikes and solar energy (which makes things warm) would initiate life. But what about the seedling?
Germination is an amazingly complex biochemical process. All seeds are little machines awaiting specific ranges of temperature, moisture, air, and light conditions to silence their dormancy genes. There must be sophisticated, robust, and efficient mechanisms already in place to detect and convert heat energy of a specific frequency into forms useful to the plant at each stage of development. Plant DNA decodes to create leaves with photosynthesizing cells which fuel activities and growth. Once the sun’s energy is captured, DNA provides additional directions throughout the plant’s life cycle, such as regulating and repairing damaged cells and DNA (see diagram)! [2]
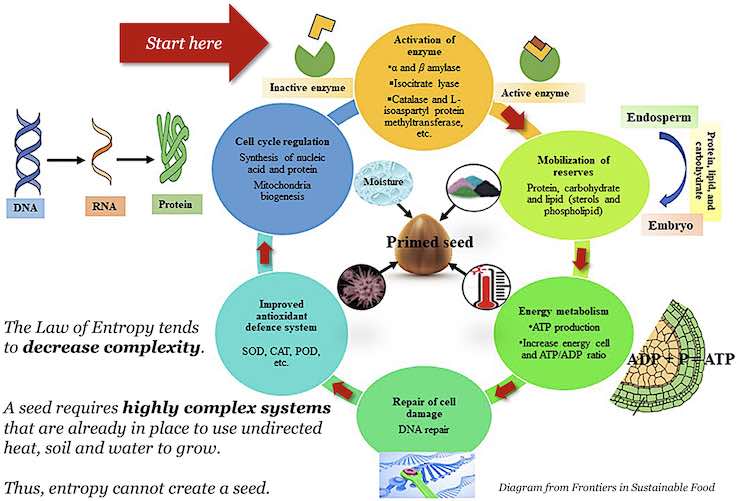
There’s nothing random about a budding plant. Without DNA’s plans, no energy is captured or effectively utilized. Without decoders, DNA’s plans are ignored. Without the right form of energy, DNA isn’t copied or read. So how could DNA’s encoded information appear in evolution’s random process? Dr. Morris describes evolution’s disconnect from the law of entropy: “Which came first, the fuel to copy the DNA code or the code for the fuel's manufacture? Evolutionary innovation is thought to occur through unguided mutation and natural selection. How many random tries would it take to either devise a complicated process (like photosynthesis) or write a complex code (like DNA)? Both must be present for life to function and continue. … The entropy law forbids them to simply appear when the need arises.” 1
The Bible affirms entropy when it says the earth “shall wax old like a garment” (Ps. 102:25-26; Heb. 1:10-12), as did Jesus who said “heaven and earth shall pass away” (literally, “is passing away”). Our world is decaying because of sin which “bringeth forth death.” Good science always aligns strongly with the Bible, not some random unproven idea. We see God’s fingerprints even in the lowly seed!
[1] Institute for Creation Research (ICR), A Barrier To Evolution, April 2010.
[2] Frontiers in Sustainable Food, O. Siva Devika, et al, Seed Priming, July 7, 2021
Like this? Consider sharing it to Facebook by clicking the linked icon below.
